|
GR0177 #53
|
|
|
|
|
Alternate Solutions |
Aleph0
2007-11-02 12:19:50 | Fortunately, the choices provided by ETS make this one relatively easy. At very low temperatures, semiconductors don't conduct (not enough thermal energy to excite electrons to the conductance band). Thus, at very low temperatures, the resistivity is very high - that means (B).
uhurulol
2014-10-20 18:40:35 |
I used the exact same reasoning here. Think about it this way; when the temperature is low in a semiconductor, electrons are less excited and thus not flowing freely, so the resistivity is high. Excite those electrons and they can flow more freely, reducing the resistivity.
|
|  |
|
|
Comments |
Quark
2011-09-26 16:01:01 | I really don't know much about this but I always thought that the lower the temperature, the higher the conductivity (i.e. the lower the resistivity). Isn't that why you always here of superconductors being chilled to close to absolute zero for the best performance?
I was also under the impression that at the higher extreme of temperature, the charge carriers possess a large amount of kinetic energy and are more likely to exhibit random motion within the conductor (either in the same direction or in the opposite direction as the current) rather than follow the electric field potential and thus contribute to the current.
cathaychris
2011-10-14 18:41:38 |
For a normal conductor, this is true... resistance increases with temperature. At very low T (think superconductors) it goes to 0. But semiconductors are the other way around.
|
maxdp
2013-10-18 12:07:45 |
I made the same mistake you did. I think the real confusion here is that the terms "semiconductor" and "superconductor" are easy to mix up. They have nothing to do with each other (and are in fact pretty much opposites). What you're describing is a superconductor, while the problem asks about semiconductors. The thing I think that's throwing you off is that rarely are you ever asked about the conductance/resistivity of a semiconductor, it's much more common to be asked about them in regards to doping. Superconductors on the other hand are all about conductance-temperature dependence. This problem is basically just checking whether you've gotten the two confused or not. Basic analysis of the idea of a semiconductor - a material that's a small energy gap away from being a conductor - should make it clear that a semiconductor at low temperature would barely conduct at all.
|
|  | mrTrig
2010-11-03 22:23:31 | How might doping effect the plot. My gut tells me it should certainly still be exponential, but why would ETS tell us that its undoped?
FutureDrSteve
2011-11-06 14:16:21 |
Undoped, or pure, semiconductors are insulators at low temperatures, and conductors at high temperatures, so the curve would look like answer choice (B).
Doping is the process of adding an impurity to a semiconductor to change the number of electrons in the outer shells in order to make it a better conductor. Doped semiconductors act more like conductors, so the curve might look a little like (A).
In conclusion, it's an important detail :P
|
|  | jondiced
2010-09-27 14:56:20 | To expand qualitatively on what herrphysik said, at higher temperatures electrons have more kinetic energy and therefore are more likely to jump across the band gap into the conduction band. At low temperatures, they do not have enough. At 0 K, for example, they cannot fill even up to the Fermi level since that is in the band gap, so you can eliminate A, C, D, and E which have non-zero conductance. |  | Aleph0
2007-11-02 12:19:50 | Fortunately, the choices provided by ETS make this one relatively easy. At very low temperatures, semiconductors don't conduct (not enough thermal energy to excite electrons to the conductance band). Thus, at very low temperatures, the resistivity is very high - that means (B).
keithpenney
2010-11-12 19:56:49 |
In general, if I'm not mistaken, doping (n-type) increases the number of charge carriers and introduces carriers within the band gap, thus minimizing the effective gap and increasing conductivity. I'm sure there's more to it than that, but that's the basic picture.
(p-type introduces positive "holes", unoccupied electronic states, which behave like positive charge carriers)
|
keithpenney
2010-11-12 19:59:17 |
This is SO not the question to which I was replying. It was a reply to "mrTrig", sorry Alepho.
|
uhurulol
2014-10-20 18:40:35 |
I used the exact same reasoning here. Think about it this way; when the temperature is low in a semiconductor, electrons are less excited and thus not flowing freely, so the resistivity is high. Excite those electrons and they can flow more freely, reducing the resistivity.
|
|  | herrphysik
2006-09-27 02:07:07 | The basic explanation for this given by Giancolli is that at higher temps, some of the electrons that are not normally free in a semiconductor become free and can contribute to the current. |  |
|
| Post A Comment! |
You are replying to:
To expand qualitatively on what herrphysik said, at higher temperatures electrons have more kinetic energy and therefore are more likely to jump across the band gap into the conduction band. At low temperatures, they do not have enough. At 0 K, for example, they cannot fill even up to the Fermi level since that is in the band gap, so you can eliminate A, C, D, and E which have non-zero conductance.
|
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces 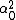 . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
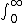 |
| $\partial$ |
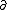 |
| $\Rightarrow$ |
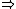 |
| $\ddot{x},\dot{x}$ |
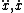 |
| $\sqrt{z}$ |
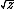 |
| $\langle my \rangle$ |
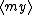 |
| $\left( abacadabra \right)_{me}$ |
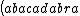_{me}) |
| $\vec{E}$ |
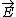 |
| $\frac{a}{b}$ |
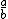 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
