|
GR0177 #17
|
|
|
Problem
|
|
|
This problem is still being typed. |
Atomic }Orbital Config }Orbital Config
First, Check that all choices have superscripts that add to 15. That's apparently so, so one can't easily eliminate choices based on that.
Since the problem has electrons in ground-state, choice (D) and (A) cannot be it--no electrons are promoted from the s to higher orbitals. s has to be filled first.
Choice (E) skips the 3s states completely, so that's out.
One should have an approximate idea of what the periodic table looks like. The transitional (d-state) elements do not occur until after 4s. Even so, the first row of transitional elements is labeled 3d. Since Phosphorous does not have enough electrons to qualify for a 4s or more state, choice (B) must be right.
|
|
|
Alternate Solutions |
| There are no Alternate Solutions for this problem. Be the first to post one! |
|
|
Comments |
potentialwell
2009-08-08 13:10:49 | as a general rule of thumb:
use the following table, crossing from bottom right to upper left, where the first arrow goes through 1s, the second goes through 2s, the third through 2p then 3 s, and so on. keep in mind s takes 2 electrons, p 6, d 10, and f 14.
.
.
.
4s 4p 4d 4f
3s 3p 3d
2s 2p
1s
nyuko
2009-11-04 22:57:36 |
as Yosun has reminded us: transitional elements occur after 4s. Your "rule of thrumb" does not work for transitional elements.
|
TheFashionCritic
2010-10-08 15:07:28 |
potentialwell's rule of thumb works, but you need to read the order if you go diagonally from the bottom right to the top left.
So you first go:
1s.
2s.
2p 3s
3p 4s
3d 4p 5s
etc.
|
gravity
2010-11-09 23:45:41 |
How have I never seen this mnemonic before?!
|
FutureDrSteve
2011-11-07 14:04:57 |
That's definitely a cool way to remember and good for the GRE. For those who are just curious, the shells go in order of increasing value of n + l, where l is given by s,p,d,f... = 0,1,2,3... When two shells such as 3d and 4p, share the same value, the lower quantum number shell is filled first. I think there are some exceptions in really large atoms, but you definitely won't encounter those on the GRE.
|
SandyBugon
2018-04-11 03:36:03 |
This image might help for a visual aid for the electronic configuration\r\n\r\nhttps://goo.gl/images/nCMQLD
|
|  | judijasa
2008-04-25 11:46:00 | I think, an easier way to see why the answer is definetly B, instead of calling the "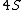 " criteria, is that before getting to the " criteria, is that before getting to the 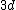 subshell you have to fill the subshell you have to fill the 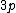 just as the just as the 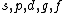 order suggests. order suggests.
judijasa
2008-04-25 11:48:14 |
Correction: the order is 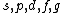 , not , not 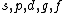
|
|  |
|
| Post A Comment! |
You are replying to:
as a general rule of thumb:
use the following table, crossing from bottom right to upper left, where the first arrow goes through 1s, the second goes through 2s, the third through 2p then 3 s, and so on. keep in mind s takes 2 electrons, p 6, d 10, and f 14.
.
.
.
4s 4p 4d 4f
3s 3p 3d
2s 2p
1s
|
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces 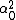 . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
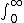 |
| $\partial$ |
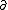 |
| $\Rightarrow$ |
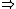 |
| $\ddot{x},\dot{x}$ |
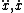 |
| $\sqrt{z}$ |
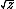 |
| $\langle my \rangle$ |
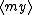 |
| $\left( abacadabra \right)_{me}$ |
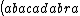_{me}) |
| $\vec{E}$ |
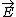 |
| $\frac{a}{b}$ |
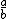 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
