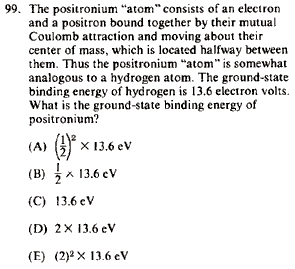
 |
Atomic }Positronium }Positronium
Interestingly, this is almost an exact repeat of Exam 9677 Prob 12. The reasoning's the same:
The positronium atom involves a positron-electron combination instead of the usual proton-electron combo for the H atom. Charge remains the same, and thus one can approximate its eigenvalue by changing the mass of the Rydberg energy (recall that the ground state of the Hydrogen atom is 1 Rydberg).
Recall the reduced mass 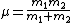 , where for identical masses, one obtains , where for identical masses, one obtains 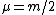 . The Rydberg in the regular Hydrogen energy eigenvalue formula . The Rydberg in the regular Hydrogen energy eigenvalue formula 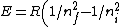) is is 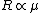 , where , where 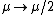 . Substitute in the new value of the reduced mass to get . Substitute in the new value of the reduced mass to get 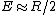 . . 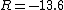 eV, and thus eV, and thus 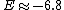 eV. eV.
|
