|
GR0177 #68
|
|
|
|
|
Alternate Solutions |
jmason86
2009-07-14 22:32:55 | The quick-thinking, time-pressure solution that I did was:
(A) 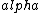 decay would change the atomic number nucleon number from 7. decay would change the atomic number nucleon number from 7.
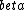 decay explains the p->n transformation but requires two particles to be emitted (e+/e- and decay explains the p->n transformation but requires two particles to be emitted (e+/e- and 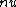 / /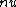 bar) bar)
(B) (C) (D) do not satisfy this
Only (E) remains. Electron capture is also basically an odd form of beta decay.. all the particles involved are the same, the equation just changes a bit. |  |
|
|
Comments |
sam16
2015-10-22 07:46:52 | The electron capture process also emits a neutrino. I don\'t see how one can guess between electron capture and beta decay in that case...\r\n\r\nSource- http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/radact2.html
sam16
2015-10-22 07:48:52 |
Edit: The options specify that explicitly. My bad!
|
|  | berri104
2013-10-14 07:57:10 | I am confused by this notation. Isn't the mass number A at the top while the atomic number Z (the number of protons) on the bottom? And how does losing a proton amount to losing a neutrino? |  | Kabuto Yakushi
2010-09-10 21:30:31 | Five particle interaction conservation laws that would probably be a good idea to memorize for this test:
1) Charge is conserved.
2) Energy is conserved.
3) Lepton number is conserved separately for each family.
4) Baryon number is conserved.
5) Strangeness is conserved for strong interaction.
As Yosun said, we see that E) is the only one that does not violate the third conservation law. |  | jmason86
2009-07-14 22:36:23 | Sorry that was sloppy. Here it is again:
The quick-thinking, time-pressure solution that I did was:
(A)  decay would change the nucleon number from 7. decay would change the nucleon number from 7.
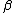 decay explains the p->n transformation but requires two particles to be emitted (e+/e- and decay explains the p->n transformation but requires two particles to be emitted (e+/e- and  / / bar) bar)
(B) (C) (D) do not satisfy this
Only (E) remains. Electron capture is also basically an odd form of beta decay.. all the particles involved are the same, the equation just changes a bit.
|  | jmason86
2009-07-14 22:32:55 | The quick-thinking, time-pressure solution that I did was:
(A) 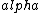 decay would change the atomic number nucleon number from 7. decay would change the atomic number nucleon number from 7.
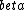 decay explains the p->n transformation but requires two particles to be emitted (e+/e- and decay explains the p->n transformation but requires two particles to be emitted (e+/e- and 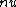 / /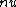 bar) bar)
(B) (C) (D) do not satisfy this
Only (E) remains. Electron capture is also basically an odd form of beta decay.. all the particles involved are the same, the equation just changes a bit. |  | Poop Loops
2008-11-01 20:25:10 | I did this by process of elimination since a particle class is only offered spring quarter senior year at my school...
So:
(A) Alpha particle is a helium nucleus. This won't get us anywhere because helium has 2 protons and we are only dropping our Z (number of protons) by one.
(B) This will simply ionize the particle, so it will still be Be, but with a positive charge. No good.
(C) This will lower the mass, but our mass stays constant here. Further, this won't lower the Z number. Nope.
(D) A positron is the anti-particle of the electron. It is exactly like the electron except it has positive charge. You don't get these in run-of-the-mill atoms, so you can throw this away. But, even if you weren't sure, you can still tell that the Z number won't go down because that refers specifically to protons.
(E) Eh? I don't know what this is. Sure, let's go with this. The little bit I do know says that Neutrons are Protons and Electrons combined together, so that helps. |  | abby
2007-10-29 10:26:09 | isn't an alpha particle a helium atom with 2neutrons and 2protons? why does the answer say 4neutrons.. (typo?)
mrbojeebers
2007-10-29 22:25:21 |
Appears to be a typo. Perhaps it was meant 4 nucleons. And not to be a stickler over niggling details, but traditionally one denotes the atomic number with letter 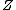 and mass or nucleon number with letter and mass or nucleon number with letter 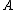 (i.e. the symbols (i.e. the symbols 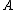 and and 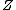 are typically swapped in your are typically swapped in your 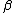 -decay equations.) -decay equations.)
|
|  |
|
| Post A Comment! |
|
|
Bare Basic LaTeX Rosetta Stone
|
LaTeX syntax supported through dollar sign wrappers $, ex., $\alpha^2_0$ produces 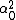 . .
|
| type this... |
to get... |
| $\int_0^\infty$ |
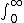 |
| $\partial$ |
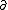 |
| $\Rightarrow$ |
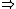 |
| $\ddot{x},\dot{x}$ |
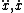 |
| $\sqrt{z}$ |
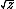 |
| $\langle my \rangle$ |
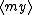 |
| $\left( abacadabra \right)_{me}$ |
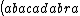_{me}) |
| $\vec{E}$ |
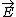 |
| $\frac{a}{b}$ |
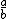 |
|
|
|
|
The Sidebar Chatbox...
Scroll to see it, or resize your browser to ignore it... |
|
|
|
